
- PERIODIC TABLE CHEMISTRY REGENTS FULL
- PERIODIC TABLE CHEMISTRY REGENTS SERIES
- PERIODIC TABLE CHEMISTRY REGENTS FREE
PERIODIC TABLE CHEMISTRY REGENTS SERIES
Key Tables and Charts include: Standard Temperature and Pressure, Physical Constants for Water, Selected Prefixes, Selected Units, Solubility Guidelines for Aqueous Solutions, Solubility Curves at Standard, Pressure, Vapor Pressure of Four Liquids, Heats of Reaction at 101.3 kPa and 298 K, Activity Series, Common Acids, Selected Radioisotopes, Common Acid–Base Indicators, Symbols Used in Nuclear Chemistry, Organic Prefixes, Homologous Series of Hydrocarbons, Organic Functional Groups, Periodic Table of the Elements, Properties of Selected Elements, and Important Formulas and Equations. Note: Sometimes this document is incorrectly spelled like 'Chemistry Refrence Table' please understand the correct document is titled ' Chemistry Reference Table' Properties of Selected Elements, Important Formulas and Equations.Ĭontains the periodic table of elements portion of the reference tables. Standard Temperature & Pressure, Physical Constants for Water, Prefixes, Units, & Polyatomic Ions, Solubility Guidelines and Curves, etc.
PERIODIC TABLE CHEMISTRY REGENTS FULL
The 2011 edition replaces all previous editions and should be used at the start of the 2011-12 school year.ĭownload FULL 2002 Chemistry Reference Table (page 1-12) Some of the tables have been moved to different pages, while others have been enlarged or replaced with updated versions. Schools are required to use the online versions to print sufficient copies to supply one reference table for each student.Ģ011 Edition of the Regents Chemistry Reference Table, has been revised to reflect the latest information on the subject. UPDATED SEPTEMBER 2011: Except for Braille and large-type editions, high school science reference tables will no longer be supplied. In a mobile friendly format (HTML5 version) 7) As each successive element in Group 15 of the Periodic Table is considered in order of increasing atomic number, the atomic radius. Smartphone Phone Users: 2011 Chemistry Reference Table
PERIODIC TABLE CHEMISTRY REGENTS FREE
The links provided below are for student use and are free and fast.

The CRT is also used on the Chemistry Regents Exam.
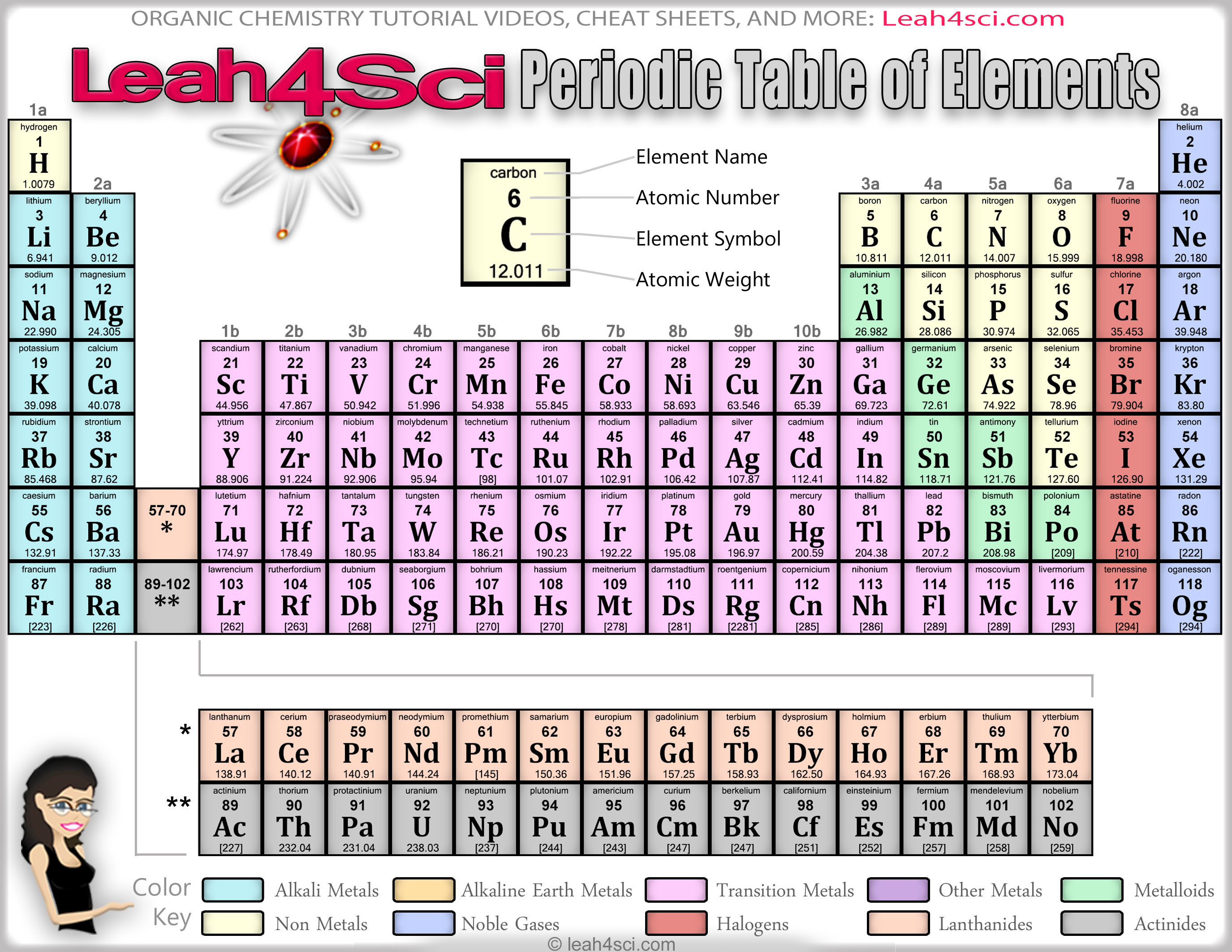
The booklet is frequently used during classes, tests, and lab assignments. It contains important measurements, equations, and identification tables. Other important groupings of elements in the periodic table are the main group elements, the transition metals, the lanthanides, and the actinides.The Chemistry Reference Tables (CRT) is an invaluable tool to the chemistry student. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable), whereas solid nonmetals are generally brittle and poor electrical conductors. They are separated by a diagonal band of semimetals. Metals are located on the left of the periodic table, and nonmetals are located on the upper right. Semimetals exhibit properties intermediate between those of metals and nonmetals. The elements can be broadly divided into metals, nonmetals, and semimetals. Some of the groups have widely-used common names, including the alkali metals (Group 1) and the alkaline earth metals (Group 2) on the far left, and the halogens (Group 17) and the noble gases (Group 18) on the far right. Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right) the seven horizontal rows are called periods. It arranges of the elements in order of increasing atomic number.

The periodic table is used as a predictive tool. As expected, semimetals exhibit properties intermediate between metals and nonmetals. Most solid nonmetals are brittle, so they break into small pieces when hit with a hammer or pulled into a wire. Nonmetals can be gases (such as chlorine), liquids (such as bromine), or solids (such as iodine) at room temperature and pressure. Nonmetals, in contrast, are generally poor conductors of heat and electricity and are not lustrous. Of the metals, only mercury is a liquid at room temperature and pressure all the rest are solids. The vast majority of the known elements are metals. Metals-such as copper or gold-are good conductors of electricity and heat they can be pulled into wires because they are ductile they can be hammered or pressed into thin sheets or foils because they are malleable and most have a shiny appearance, so they are lustrous. The distinction between metals and nonmetals is one of the most fundamental in chemistry.

Gold-colored lements that lie along the diagonal line exhibit properties intermediate between metals and nonmetals they are called semimetals. \( \newcommand\) divides the elements into metals (in blue, below and to the left of the line) and nonmetals (in bronze, above and to the right of the line).


 0 kommentar(er)
0 kommentar(er)
